Keywords
electrodeposition, electrochemistry, cathode, cathodic deposit, anode, electrochemical cell, moving mesh, uniformity, metal growth
Context / Goal
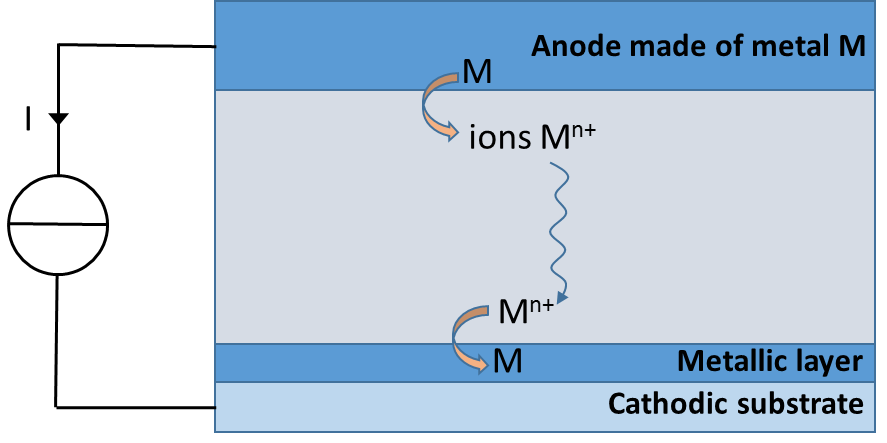
Electrodeposition is an electrochemical process in which a metal layer is deposited on a substrate. There are numerous applications of this process from a simple aesthetic layer to a functional material used, for example, in electrical circuits. The electrochemical cell is comprised of a cathode (substrate), an anode made of the metal to be deposited and the electrolytic solution with the metallic ions. When a current is prescribed, the metal which constitutes the anode is oxidized into ions in the electrolyte. These ions migrate toward the cathode where they are reduced back into a metal, forming the layer (see illustration). The main requirement is to obtain a layer with the most uniform thickness possible.
Schema of the electrodeposition method
In this example, the growth of a copper layer onto an electrical circuit made of slots is being simulated. The main objective is to predict the thickness profile of the copper as a function of the current applied to the cell.
SIMTEC's Achievements / Results
The model developed is an electrical model of the tertiary currents. It takes into account :
-
the ohmic drops in the electrolyte,
-
the reaction kinetics for deposition and dissolution of copper,
-
the effects of the electrolytic concentration of Cu2+ on the reaction, and
-
the increase of the copper thickness at the cathode as well as the decrease of the anode thickness, by use of the moving mesh method.
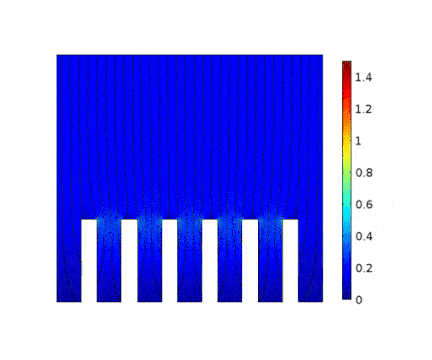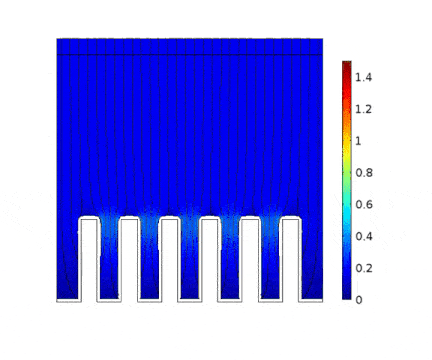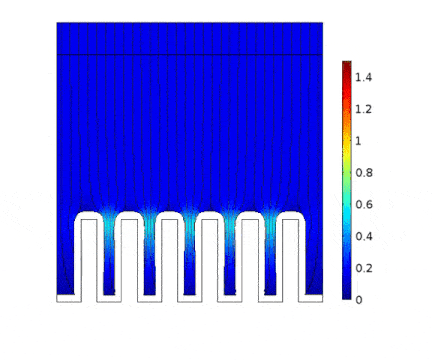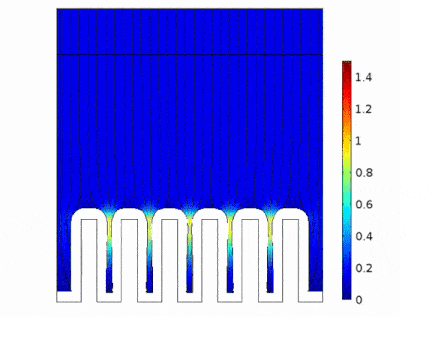
The process is modeled for two different current solicitations, differing from one another by a factor of 5 (0.15 and 0.8 A/cm² at the anode).
The main consequences of using a high current are :
-
Presence of huge edge effect at the top of the slots, associated with high current densities.
-
Heterogeneous growth of copper with early obturation of slots as a consequence of the edge effects. On the contrary, with a weak current, copper growth with a much more uniform thickness.
-
Depletion of the copper ions in the holes due to the increase of the deposition velocity and, as a consequence, to the increase of the Cu2+ consumption rate.
| Low current | High current |
 |
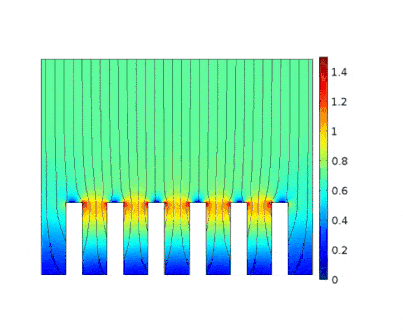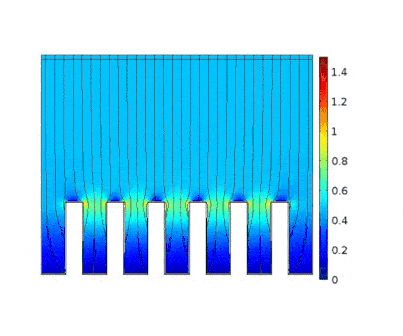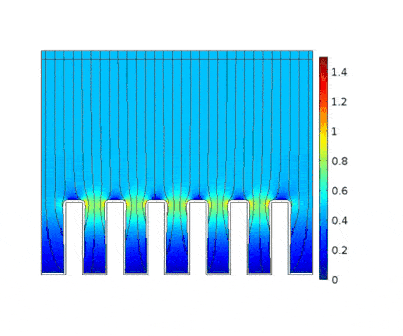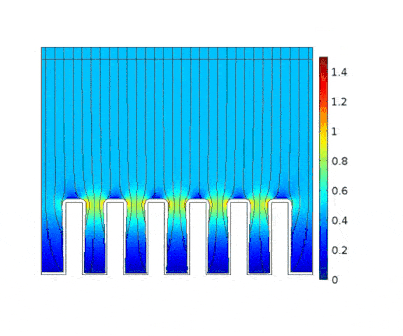 |
This model can be used to choose an optimal deposition current for which the metal layer will be uniform enough and the deposition rate viable for industrial application.